Between August 1991 and March 1992, 96,000 women aged 29 to 49 years residing in the Uppsala region in Sweden were randomly selected from the Swedish Total Population Register and invited to participate in the WLH Study27. Among those invited, 49,260 (51%) women consented to be a part of this study, and returned the baseline questionnaire, which included a food frequency questionnaire (FFQ). The median age at enrollment was 40 years. Follow-up began at the date of returning the baseline questionnaire. We followed women until the date of PD, AD, or ALS diagnosis, emigration from Sweden, death, or March 31st, 2022, whichever occurred first. We used the Swedish National Patient Register to identify incident cases of PD, AD, and ALS, and the Total Population Register to identify emigration and death, through cross-linking the WLH Study and these registers using unique Swedish personal identity numbers. The study was approved by the Swedish Ethical Review Authority (DNR: 2020-02976). Informed consent to participate in the study was collected from all study participants.
Exposure assessment
Participants were asked to recall the frequency and quantity of 80 foods and beverages consumed over the previous six months before cohort entry. The total consumption (g/day) of foods and drinks were summarized, and intakes of energy (kJ/day), and nutrients were calculated by linkage to the Swedish National Food Administration database49. Further, we calculated adherence to an MDP using the scale developed by Trichopoulou et al.10 as in other studies using the WLH Study20,50.
An MDP was calculated based on the following nine food groups: vegetables, fruits and nuts, cereals, legumes, dairy products, fish and seafood, meat, alcohol, and monounsaturated-to-saturated (M/S) fat ratio. All participants were scored on each food group based on the median consumption of the entire cohort (Supplemental Table 5). Consumption greater than or equal to the cohort median for beneficial food groups, including vegetables, fruits and nuts, cereals, legumes, fish and seafood, and high M/S fat ratio, was scored as 1, where consumption less than the cohort median was scored 0. Conversely, non-beneficial food groups, dairy products and meat, were scored in the reverse direction. Alcohol was scored separately, where moderate level of consumption (5–25 g/day) was scored as 1, and greater than or less than moderate consumption was scored 0. Scores among all nine food groups were summed, yielding a score of MDP adherence ranging from 0 (least adherent) to 9 (most adherent). This score was also categorized into low (score 0–3), moderate (4–5), and high (6–9) adherence10.
Outcome assessment
The Swedish National Patient Register was used to identify the first clinical diagnosis of the studied neurodegenerative diseases, where nationwide data on inpatient care was available from 1987 and outpatient care was available from 200151. The following codes of the Swedish revisions of the International Classification of Disease (ICD) were used to define PD (ICD-9: 332 A, 333 A, ICD-10: G20, F023, G214, G218, G219, G231, G232, G239, G259, G318A), AD (ICD-9: 290 A, 290B, 331 A, ICD-10: F00, G30), and ALS (ICD-9: 335 C, ICD-10: G12.2). Using ICD codes to identify neurodegenerative diseases based on the National Patient Register has been validated against clinical diagnosis, demonstrating high specificity for PD ( > 98%)52 and AD (99.7%)53, and a high positive predictive value for ALS (91%)54.
Covariates
Questionnaires administered at baseline also collected information on demographic characteristics, lifestyle factors, and medical history. We included several variables as potential confounders in the association between MDP and PD, AD, and ALS, including year of birth (1942–1946, 1947–1951, 1952–1956, 1957–1962), body mass index (BMI in kg/m2, <25, ≥25 and <30, ≥30), years of education (0–10, 11–13, >13), level of physical activity (self-reported levels: very low, low, moderate, high, very high), smoking status (never, former, current), and total energy intake (kJ/day) calculated through the Swedish National Food Administration database49. Since the presence of a morbidity may influence dietary intake and the future risk for neurodegenerative diseases, we additionally included self-reported medical history of diabetes or hypertension (yes, no) at baseline as covariates.
Statistical analysis
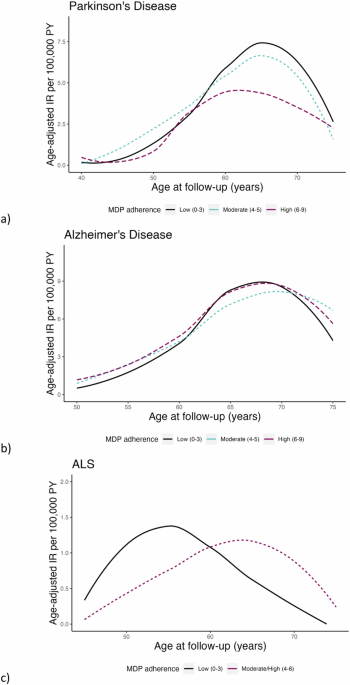
Age-adjusted incidence rates (IR) were calculated for PD, AD, and ALS separately, standardized using the distribution of attained age during the follow-up of the entire cohort. Additionally, age-adjusted IR for PD, AD, and ALS were calculated in 5-year age intervals by MDP adherence and visualized using a loess smoothing curve over age at follow-up. The association between MDP and PD, AD, and ALS was assessed using Cox regression models to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs), using attained age (age at follow-up) as the underlying time scale. This MDP was evaluated as both a categorical (0–3 low, 4–5 moderate, 6–9 high) and continuous (0–9, per unit increase) variable. In a minimally adjusted model, we adjusted for year of birth, and in the fully adjusted model we additionally adjusted for BMI, education, physical activity, smoking status, history of diabetes and hypertension, and total energy intake as defined above. Natural cubic splines were fitted to assess the risk of PD, AD, and ALS across MDP score as a continuous variable (0–9), using an MDP score 4 as the reference. The proportional hazards assumption was assessed through the standardized Schoenfeld residuals for each outcome.
Since older age is a common risk factor across these neurodegenerative diseases, we fitted the Cox regression models in sub-groups of attained age <60 and ≥60 years according to the findings of age-adjusted IR over age at follow-up and upon evaluation of the Schoenfeld residuals. In the age-stratified analysis of ALS, we collapsed the high and moderate adherence MDP levels to account for reduced numbers of ALS cases.
To alleviate the concern of potential reverse causation, we removed the first two or the first five years of follow-up from the analysis in a sensitivity analysis.
Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC). All tests of statistical hypotheses were done on the two-sided 5% level of significance. This study followed the STROBE (Strengthening the reporting of observational studies in epidemiology) guidelines for cohort studies (Supplemental Table 6).