Chemicals and enzymes
All chemicals and reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzymes used included lipase (100–500 U/mg protein), alpha-amylase (1.5 U/mg), pepsin (≥ 250 units/mg solid), pancreatin (8×USP), and mucin. The salts and buffers utilized in the experiments comprised NaCl, KCl, CaCl2·2 H2O, NaHCO2, and urea. Bovine serum albumin (BSA) was used as a protein standard. The solvents and reagents included glyoxal (40%), methylglyoxal (40%), methanol, acetonitrile (ACN), sodium acetate, 4-nitro-1,2-phenylenediamine, meta-phosphoric acid, thiobarbituric acid, trichloroacetic acid (TCA), and bile salts mixture.
Preparation of samples
Beef round and chicken breast meat were used in the study. Meats were purchased from a local supermarket in Türkiye. Seven types of beverages (freshly squeezed orange juice, 100% grape juice, red wine, hardaliye (a fermented grape drink), shalgam (fermented black carrot juice), freshly squeezed pomegranate juice, and ayran) and three types of salads (Mediterranean salad, coban salad, and red cabbage salad) were used for co-digestion with the meat. Salads, orange juice, and pomegranate juice were freshly prepared on the analysis day, while other beverages were purchased from a local supermarket in Türkiye. Mediterranean salad contained 50 g of carrot, 50 g of iceberg lettuce, 10 g of corn, 30 g of tomato, 2 g of fresh basil, juice of 50 g lemon, 5 g of vinegar, 5 g of extra virgin olive oil, and 0.5 g of salt. Coban salad contained 30 g of green pepper, 50 g of cucumber, 20 g of onion, 150 g of tomato, 2 g of fresh basil, 2 g of fresh mint, juice of 50 g lemon, 5 g of extra virgin olive oil, and 0.5 g of salt. Red cabbage salad contained 50 g of carrot, 200 g of red cabbage, 2 g of fresh basil, juice of 50 g lemon, 10 g of vinegar, 5 g of extra virgin olive oil, and 0.5 g of salt. The meats were cooked on an electric grill (Kenwood HG230, 2200 W, Kenwood, UK) until the internal temperature reached 75 °C, then blended using a blender (Philips, Netherlands) and vacuum packed using a vacuum sealer (Vestel, Türkiye) to prevent exposure to oxygen. Samples were stored at − 20 °C until analysed. The amounts of meat and beverages used for in vitro digestion were determined based on standard portion sizes outlined in the National Nutrition Guide of Türkiye to ensure physiological relevance. One portion of meat (80 g) was prepared for co-digestion with one portion of beverage (200 mL) or one portion of salad (150 g). The final sample mixtures consisted of 5 g of meat combined with either 12.5 mL of beverage or 9.4 g of salad, ensuring consistency across all experimental groups.
To ensure the freshness of the meat samples, their delivery date was inquired at the supermarket and selected the freshest available products. All meat samples used in the study were sourced from the same batch to maintain consistency. Following the cooking process, the samples were homogenized through blending, ensuring uniform composition and standardized storage and processing conditions across all experimental groups.
In vitro digestion
The in vitro gastrointestinal digestion was performed according to the INFOGEST® 2.0 protocol20, with modifications to the specific conditions proposed by Lee et al.21. The preparation of mouth, stomach, small intestine, and bile solutions were shown in Fig. 1.
Fig. 1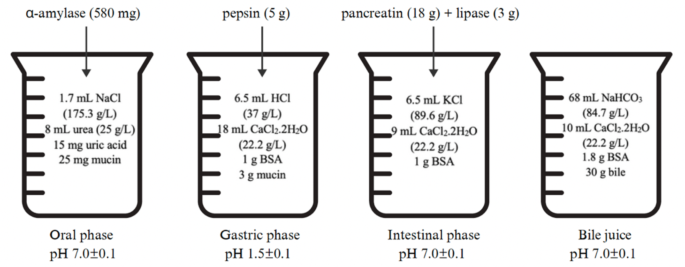
In vitro human digestion system procedure.
In this study, 5 g of each meat sample was co-digested with 12.5 mL of beverages and 9.4 g of salads. The samples were homogenized using a homogenizer (IKA® T18 Digital Ultra Turrax, IKA, Germany) with 5 mL of saliva juice and incubated for 5 min at 37 °C in a shaking water bath (Memmert, Germany). Subsequently, 12 mL of gastric juice solution was introduced, and the samples were incubated for 30 min at 37 °C in the same conditions. To simulate intestinal conditions, 5 mL of bile solution and 10 mL of duodenal juice were added, the pH was adjusted to 7, and the mixture was incubated for 2 h at 37 °C. The reaction was then halted by adding 10% TCA, and the volume was adjusted to 50 mL using deionized water. The samples were centrifuged at 10,000 rpm for 10 min at 22 °C using a centrifuge (Himac CR22N, Hitachi, Japan), filtered through a 0.45 μm cellulose acetate filter, and then injected into the HPLC system for analysis.
Malondialdehyde analysis
MDA determination was based on the modified methods of Bertolín et al.22, and Zhang et al.23, with tetraethoxypropane used to prepare the MDA standard. A stock solution was prepared by adding 0.5 mL of tetraethoxypropane to a 100 mL volumetric flask and diluting with ethanol, with each standard completed using 10% TCA. For each meat sample, 30 mL of 10% TCA solution was added to 50 mL falcon tubes, and the samples were homogenized, at 13,000 rpm for 1 min at 4 °C using a homogenizer (IKA® T18 Digital Ultra Turrax, IKA, Germany), followed by centrifugation at 10,000 rpm for 2 min at 22 °C using a centrifuge (Himac CR22N, Hitachi, Japan). The volume was adjusted to 50 mL with TCA, and a second centrifugation was performed at 15,000 rpm for 10 min at 22 °C. Then, 1 mL of the supernatant was reacted with 1 mL of 0.67% thiobarbituric acid solution, heated at 90 °C for 30 min, cooled at room temperature, filtered through a 0.45 μm cellulose acetate filter, and injected into the HPLC system.
Glyoxal and methylglyoxal analysis
Determination of GO and MGO was based on the modified methods of Cengiz et al.24. The sample was placed in a 50 mL falcon tube and 25 mL of methanol was added. The sample was homogenized for 1 min at 4 °C using an ultra-thorax homogenizer (IKA® T18 Digital Ultra Turrax, IKA, Germany) and then centrifuged at 8000 rpm for 5 min at 22 °C using a centrifuge (Himac CR22N, Hitachi, Japan). 0.5 mL of the centrifuged supernatant was transferred to a glass tube and phosphate buffer (pH = 3) was added. Then 0.5 mL of 4-nitro-1,2-phenylenediamine solution (50 mg/50 mL methanol) was added for derivatisation and incubated in a water bath at 70 °C for 30 min. It was then filtered through a 0.45 μm cellulose acetate filter and injected into the HPLC.
HPLC parameters
The HPLC was consisted of a Shimadzu LC-20AT pump coupled with a Shimadzu SPD-20 A UV/VIS detector (Shimadzu Corporation, Kyoto, Japan). The separation was performed on an Inersil ODS-3 C18 column (4.6 mm × 250 mm, 5 μm particle size) at a column temperature of 30 °C. The mobile phase was composed of 0.05 M KH2PO4 buffer solution/methanol/acetonitrile (72/17/11, v/v/v), and the flow rate was maintained at 1 mL/min. A fluorescence detector was used with an excitation wavelength of 530 nm and an emission wavelength of 550 nm. The injection volume was 10 µL, and the system operated under an isocratic elution mode with a total run time of 15 min.
Calculation of inhibition and promotion
The percent MDA, GO, MGO inhibition by salads and beverages was calculated using Tang et al.‘s formula25.
$$\text{Increase decrease} \; \% = (\text{after digestion}- \text{before digestion})/\text{before digestion} \times 100.$$
$$\text{Inhibition or promotion} = (\text{after digest with salads}-\text{after digestion meat only})/\text{after digestion meat only} \times 100.$$
Statistical analysis
All analyses were performed in triplicate, with data presented as mean ± SD. ANOVA with Tukey’s post-hoc test (Minitab v15) was used for multiple comparisons, considering p < 0.05 as significant.
