Study design and population
Data were taken from the Study on Nutrition and Cardiovascular Risk in Spain (ENRICA), whose methods have been reported elsewhere [27]. In brief, 12,948 individuals ≥18 years old were selected between June 2008 and October 2010 by random stratified cluster sampling to ensure a representative sample of the non-institutionalized Spanish population. First, the sample was stratified by province and municipality size. Second, the clusters were randomly selected in two stages: municipalities and census sections. Finally, the households within each section were selected by random telephone dialing using the telephone directory as the sampling frame. Participants in the households were selected proportionally to the sex and age distribution of the Spanish population. During the first telephone call, the overall objectives and procedures of the study were explained, and selected individuals provided initial consent to participate; a formal letter of invitation and detailed written information on the study characteristics were sent to the participant’s home. Collection of blood and urine samples were included for participants’ acceptance, and the overall response rate was 51.5%. Among those not participating, the more frequent reasons were refusal to agree to a blood extraction (51.7%), no interest in the study (37.8%), and lack of time to participate (10.7%) [27].
The study was approved by the Clinical Research Ethics Committees of the La Paz University Hospital in Madrid and the Hospital Clinic in Barcelona (Spain). Written informed consent was obtained from all participants.
Baseline data collection
At baseline (during the years 2008–2010), data on sociodemographic, lifestyle such as hours watching TV and physical activity [28], as well as morbidities were collected. Self-reported information was obtained on sex, age, educational level (no formal education, primary, and secondary or higher), and tobacco consumption (current, former, and never smoker). Weight and height were measured at home under standardized conditions, and body mass index (BMI) was calculated. The number of medications were checked against drug packages. Hypertriglyceridemia was defined as fasting plasma triglycerides ≥150 mg/dL; hypercholesterolemia as fasting plasma total cholesterol level ≥200 mg/dL or taking lipid-lowering medications; high blood pressure was defined as ≥140/90 mmHg or taking antihypertensive medication; and diabetes as self-reported or taking diabetes medication. Finally, self-reported physician-diagnosed chronic conditions (chronic obstructive respiratory disease, coronary heart disease, stroke, heart failure, osteoarthritis, cancer, and depression requiring treatment) were also collected.
Dietary assessment
Trained and certified personnel collected information in three sequential stages: (1) a telephone interview to obtain data on sociodemographic factors, health behaviors, self-rated health, and morbidity; (2) a first home visit to collect blood samples, and (3) a second home visit to perform a physical examination, and to obtain habitual diet by using a computerized dietary history [27].
To ascertain the participant’s habitual food consumption, we used a validated computer-based dietary history (DH-ENRICA), developed from that used in the Spanish EPIC cohort [29]. It consists of a structured questionnaire administered by a trained interviewer following each eating occasion, from breakfast to just before bedtime. In the interview, respondents were asked about food consumption during the week and on the weekend, as well as seasonal variations. The DH-ENRICA collects standardized information on 880 foods and 184 recipes for dishes commonly eaten in Spain. Spanish standard food composition tables allowed for the calculation of the amount of energy and nutrients consumed [30, 31]. Study participants reported how often they had consumed different types of oils and fats, and they specified the type of oil used for cooking and dressings, as well as the oil that was part of recipes and sauces. In particular, detailed data were obtained on the consumption of common and virgin OO—comprehensively considering the dressing and cooking and frying methods.
We also calculated the Mediterranean Diet Score, based on the definition proposed by Trichopoulou et al. [32, 33] where the consumption of vegetables, legumes, fruits and nuts, cereals, and fish was considered beneficial. A value of 1 was assigned to subjects with consumption above the sex-specific median in the study sample. In contrast, consumption of red meat, processed meat and poultry, and dairy products was considered detrimental, and a value of 0 was assigned to consumption above the median. Two items, alcohol consumption (to be able to adjust the models independently for alcohol consumption without over-adjusting) and the ratio of monounsaturated/saturated fatty acids (because OO consumption is the main source of monounsaturated fatty acids (MUFAs) in the Spanish population) were not included in the index. The range of this modified index was 0 (lowest adherence) to 7 (highest).
Mortality ascertainment
For all-cause mortality, we used the Spanish National Death Index that contains information on the vital status of all residents in Spain. Data for specific cause of death were obtained from the Statistics National Institute of Spain (https://www.ine.es/en/index.htm). All-cause deaths were obtained from baseline in 2008–2010 to the end of follow-up on January 31, 2020, while those from CVD or cancer were obtained from baseline to January 31, 2017. Follow-up was censored at the date of death or at the end of follow-up, whichever occurred first.
Statistical analysis
Out of 13,105 participants, after excluding those that reported extreme total energy intake (800 or 5000 kcal/day for men and 500 or 4000 kcal/day for women [34]) (n = 884) and those with incomplete baseline dietary data (n = 60), a total of 12,161 participants were included in the present analysis (5708 men and 6346 women, mean age: 47 ± 17 years old).
Total OO consumption (in g per day) was estimated by adding the common and virgin varieties. OO consumption was adjusted for total energy intake by the residual’s method [35] and participants were categorized according to sex-specific tertiles of total, common, and virgin OO consumption.
To assess the associations between OO consumption and all-cause, cardiovascular and cancer mortality, Cox proportional hazard models were fitted, with attained age as the underlying timescale (birth date as origin). Hazard ratios (HRs) and their 95% confidence intervals (CI) were calculated using the lowest tertile of OO consumption as a reference or considering OO consumption as a continuous variable (per each 10 g/day, ~1 tablespoon). To investigate linear trends across tertiles of OO consumption we assigned the median value to each category and considered the variable as continuous.
We adjusted the Cox regression models for several potential confounders defined “a priori” and selected according to previous causal knowledge [36]. Thus, three models were built with progressive levels of adjustment for confounders based on information collected at enrollment. Model 1 included sex and age (continuous) and total energy intake (kcal/day). Model 2 was further adjusted for educational level, smoking status, BMI (<25, ≥25–<30, and ≥30 kg/m2), total physical activity (household and leisure-time activities in METs-hour/week), watching TV (hours/day), alcohol consumption (g/day), fiber intake (g/day), Mediterranean Diet Score (continuous from 0 to 7), number of medications (0, 1–3, and >3). Lastly, model 3 was also adjusted for possible mediators of the association of interest, i.e., hypertriglyceridemia (yes/no), hypercholesterolemia (yes/no), hypertension (yes/no), diabetes (yes/no), number of self-reported chronic conditions (0, 1, and ≥2). When assessing separately common and virgin OO consumption, models were further mutually adjusted. When missing values were <1% for individual covariates we used stochastic regression (which adds a random error term that appropriately reproduces the correlation between X and Y) to impute the data. All results were checked against models with complete information for all variables.
Restricted cubic spline analyses with 3 knots (at the 10th, 50th, and 90th percentiles) adjusted for the same potential confounders were represented to visually display the dose-response relationship between the consumption of total, common and virgin OO and mortality risk.
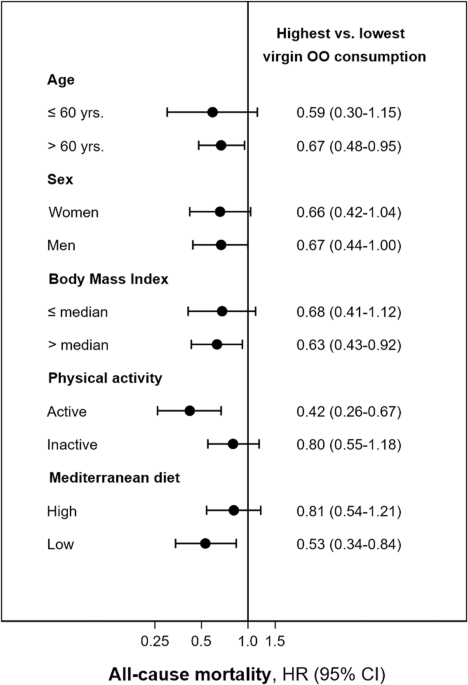
We re-ran the models for total and cardiovascular mortality including or excluding participants already diagnosed with CVD or diabetes at baseline. In addition, subgroup analyses were performed for all-cause mortality, stratifying the sample (above or below the median) by possible effect modifiers, such as age (≤ or >60 years), sex, BMI (≤ or >26.3 kg/m2), physical activity (≤ or >61.5 METs-h/week), as well as the adherence to the MedDiet (≤ or >score 3). P for interaction was obtained using the likelihood ratio test of the models with and without the interaction term. Finally, sensitivity analyses were performed after excluding the first 2 years of follow-up.
Analyses were carried out using STATA/SE version 16.0 (StataCorp, College Station, TX, USA). Analyses were weighted by using the svy Stata command to account for the complex sampling design. P values were two-tailed and p < 0.05 was considered as statistically significant.