This study provides new evidence on the association between SCFAs and the risk of developing T2D. Elevated baseline circulating concentrations of butyrate and isobutyrate were identified as independent predictors of T2D incidence over a median follow-up period of seven years. These associations remained significant after adjustment for traditional risk factors, including age, sex, family history of T2D, prediabetes, obesity, hypertension, and dyslipidemia. The findings of this study are consistent with the established role of SCFAs as key metabolites produced by the gut microbiota through the anaerobic fermentation of dietary fibers. Previous research has underscored the critical role of SCFAs in maintaining gut health and influencing systemic metabolic processes [4]. This study builds upon existing knowledge by identifying an association between SCFAs and the risk of T2D, emphasizing their potential utility as biomarkers for disease prediction.
Our findings further demonstrate a positive association between the consumption of high-fiber foods at baseline and baseline circulating concentrations of SCFA, which agrees with previous studies that identify dietary fibers as the primary substrates for SCFA production [4]. In this study, higher intake of high-fiber foods at baseline was associated with higher baseline circulating concentrations of acetate, propionate, butyrate, and isobutyrate, emphasizing the critical role of dietary patterns in modulating SCFA levels. Moreover, adherence to the MedDiet has been linked to elevated SCFA concentrations and inversely associated with markers of intestinal permeability, such as lipopolysaccharide-binding protein and zonulin, suggesting that SCFAs may serve as a mechanistic link between diet and the maintenance of intestinal barrier integrity [22]. Notably, while baseline circulating concentrations of acetate increased with the increase of the adherence to the MedDiet at baseline, other SCFAs did not exhibit a similar pattern, indicating that specific dietary fibers may differentially influence SCFA production [22]. This differential effect may be attributed to the distinct fermentation properties of individual fibers, their interactions with specific gut microbiota compositions, and the varying affinities of SCFAs for their receptors, such as FFA2 and FFA3, as suggested by Ulven et al. [5]. These findings highlight the complexity of the interplay between dietary fibers, SCFA production, and gut microbiota.
Observational epidemiological and interventional clinical studies indicate that both fiber consumption [23,24,25,26] and the adherence to a MedDiet [27,28,29] are causally related to a higher risk of T2D. Nevertheless, although there is an association between the dietary patterns and the circulating concentrations of SCFA, we did not find any association between the consumption of high-fiber foods and the adherence to MedDiet with T2D prediction, which is probably a paradoxical result. However, we found an association between baseline consumption of high-fiber foods and incident T2D when performing the sensitivity analyses (excluding subjects with prediabetes). In this sense, some authors have suggested that the effect of SCFA on the substrate and energy metabolism may differ between metabolic phenotypes [30,31,32]. Canfora et al. demonstrated that supplementation of a fiber mixture increased distal colonic bacterial fermentation in lean individuals, which improved metabolic health parameters. However, in subjects with overweight/obese and prediabetes, the supplementation with a fiber mixture increased circulating acetate concentrations. Still, it did not improve metabolic health parameters [33]. Therefore, the observed differences in behaviour between healthy people and those with prediabetes may be consistent with the findings presented in this study.
The potential of butyrate and isobutyrate as predictive biomarkers for T2D is particularly compelling. Both baseline circulating concentrations of SCFAs demonstrated strong predictive capabilities for T2D, with C-statistics comparable to traditional clinical risk factors. However, their inclusion in a conventional risk model resulted in only marginal improvements in predictive accuracy, which were not statistically significant. Despite this, both baseline circulating concentrations of SCFAs significantly enhanced risk prediction compared to the FINDRISK Score, emphasizing their potential clinical utility. These observations align with those of Husted et al. [2], who highlighted the role of metabolites as signaling molecules capable of modulating metabolic pathways and influencing disease outcomes. Integrating SCFAs into existing predictive models could offer a more refined assessment of T2D risk, particularly for individuals with borderline or ambiguous clinical profiles. Such an approach underscores the relevance of these metabolites as complementary biomarkers, providing additional insights beyond those offered by traditional risk factors [2].
Although the bulk of evidence suggests that increased SCFA production benefits the host by exerting antiobesity and antidiabetic effects [34, 35], some in vitro and in vivo studies have indicated that overproduction or accumulation of SCFAs in the bowel may also lead to obesity or T2D, owing to increased energy accumulation, higher capacity to harvest energy through SCFA production or increased de novo lipogenesis [9, 36,37,38]. In our study, baseline circulating concentrations of SCFA were higher in individuals with pre-existing T2D and those who developed T2D during the follow-up period. This observation aligns with prior studies [9, 36], which reported elevated fecal SCFA levels in overweight, obese, and subjects with T2D. However, focusing on circulating concentrations of SCFA offers a more precise representation of in vivo fermentation processes, as fecal SCFA levels may not fully capture the systemic metabolic state. Elevated baseline circulating concentrations of butyrate and isobutyrate, in particular, may signify an adaptive response to the metabolic dysregulation associated with T2D, highlighting the intricate interactions between gut-derived metabolites and host metabolism. These findings underscore the need for further research into the underlying mechanisms of this relationship.
Additionally, the results are consistent with Zhao et al. findings, who observed a significant increase in SCFA and zonula occludens-1 concentrations (used for assessing tight junctions integrity and regulation of intestinal permeability) in subjects with T2D [39]. The authors hypothesised that the intestinal barrier destruction, driven by hyperglycemia, increased the passive reabsorption of SCFAs, which resulted in the rising circulating concentrations of SCFA in subjects with T2D. Thus, the study suggested that excessive SCFA absorption may occur via a leaky gut caused by gut microbiota dysbiosis, leading to higher levels of circulating SCFAs in subjects with T2D and that the leaky gut might be caused by the disordered gut microbiota [39]. These observations further emphasize the role of SCFAs in metabolic dysregulation and gut barrier integrity, providing important insights into the pathophysiology of T2D.
The association of SCFAs with the development of T2D underscores the pivotal role of gut microbiota and their metabolic products in maintaining metabolic health. The independent predictive value of butyrate and isobutyrate warrants further investigation into the mechanistic pathways through which these SCFAs influence glucose metabolism and insulin sensitivity. The primary fermentation products resulting from digestion of dietary fibre in the intestine are SCFA, which are widely recognized for their role as key energy sources in metabolic pathways. Normal colonic epithelia obtain 60–70% of their energy supply from SCFA, particularly butyrate. The liver primarily absorbs propionate, serving as a precursor for gluconeogenesis, liponeogenesis and protein synthesis. Acetate enters systemic circulation to be metabolised by peripheral tissues and acts as a substrate for cholesterol synthesis. An excess production of SCFA from dietary compounds, potentially caused by alterations of gut microbiota, may escape digestion in the small intestine, therby providing the host with an additional energy source [9].
However, the potential of SCFAs to act as extracellular signalling molecules also offers a plausible explanation for their involvement in modulating metabolic and inflammatory responses, which are key contributors to T2D pathogenesis [3]. Notably, SCFAs, particularly butyrate, are known to activate GPCRs such as FFA2 and FFA3, which are expressed in several tissues, including human colonic tissues, as well as pancreatic islets, adipose tissue, skeletal muscle, liver, and immune cells. These receptors mediate various metabolic and immune processes, suggesting that SCFAs may influence T2D development through multiple mechanisms [3, 6, 7]. Observational and interventional studies have shown that SCFAs affect a range of host systems and energy homeostasis by activating GPCRs, preserving intestinal integrity and barrier function, influencing immune regulation and inflammatory responses (by the reduction of the secretion of pro-inflammatory cytokines and chemokines), and modulating the enteric nervous system and brain-gut axis. Furthermore, SCFAs may play crucial roles in maintaining glucose homeostasis and insulin sensitivity by: 1) in pancreatic islets, by regulating β-cell insulin secretion; 2) in adipose tissue, by enhancing lipid buffering capacity, increasing the oxidation of fatty acids in brown adipose tissue, facilitating the browning and mitochondrial function of white adipose tissue, diminishing the size of adipocytes and by the inhibition of endogenous lipolysis within adipocytes; 3) in the liver, by decreasing glycolysis and gluconeogenesis, and increasing glucose uptake and glycogen synthesis and fatty acids oxidation and improving mitochondrial function; and 4) in the skeletal muscle by improving glucose uptake and by the inhibition of ectopic lipid storage in skeletal muscle [40, 41]. Additionally, the role of SCFAs in modulating incretin hormones, essential to maintain glucose metabolism, offers further insights into the connection between gut microbiota-derived metabolites and T2D. SCFAs can also stimulate the local release of satiety hormones such as GLP-1 and peptide YY (PYY) from enteroendocrine L-cells via the activation of GPR41 or GPR43 [40, 41], while in circulation, they epigenetically regulate the expression of adipokines such as leptin, adiponectin, and resistin. Recent evidence indicates that circulating SCFAs are associated with GLP-1 concentrations, whole-body lipolysis, and peripheral insulin sensitivity in humans [42]. This emphasizes the therapeutic potential of targeting gut microbiota and their metabolites in T2D prevention and management.
Recent evidence indicates that SCFA may exert divergent effects depending on the metabolic context. It has been hypothesized that intestinally produced SCFAs are processed differently in insulin-resistant states, potentially influencing glucose and lipid metabolism [43]. Tang et al. demonstrated that, under diabetic conditions, elevated SCFA concentrations interact with FFA2 and FFA3, impairing glucose-stimulated insulin secretion. Furthermore, they suggested that antagonists targeting FFA2 and FFA3 may enhance insulin secretion in people with T2D [44]. These findings highlight the bidirectional relationship between gut microbiota-derived SCFAs and T2D, underscoring the complexity of this emerging field. Despite these advancements, numerous questions remain unanswered, necessitating further research to elucidate the role of SCFAs in glucose regulation in T2D.
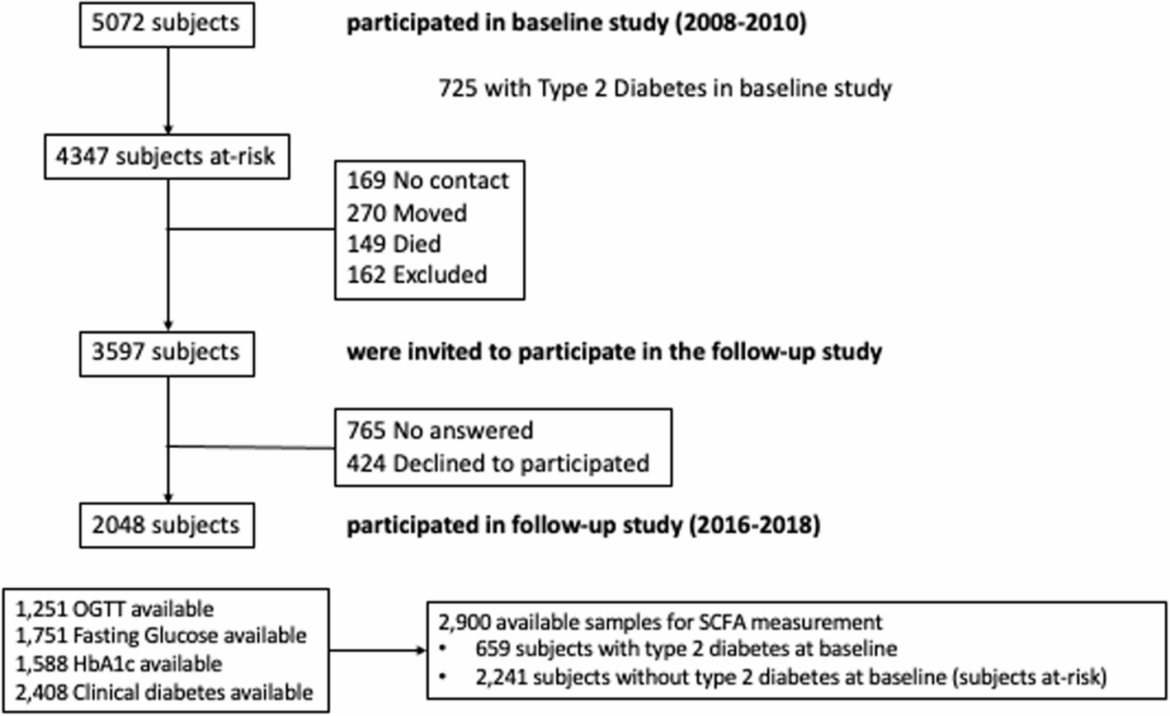
Although this study offers valuable insights, certain limitations should be acknowledged. As previously explained, the Di@bet.es study was the first nationwide, prospective, population-based study in Spain designed to determine the prevalence and incidence of T2D [10, 11]. Subjects were assessed at baseline (prevalence study) and a second time for the follow-up study (incidence study) but were not followed periodically between these two time points. Thus, as patients were evaluated only at the start and end of the study (after 7 years), the exact time of our endpoint (new cases of T2D) was unknown. The absence of gut microbiota analysis, resulting from the unavailability of fecal samples, restricts the ability to directly associate specific bacterial populations with SCFA production and the risk of T2D. In addition, we could not differentiate dietary vs. host-derived SCFA sources. Thus, the absence of gut microbiota data and the inability to differentiate dietary vs. host-derived SCFA sources limits our capacity to explore deeply the mechanistic insights of the role of SFCA in the development of T2D. Furthermore, the high attrition rate observed in the follow-up cohort (45%) raises concerns about potential selection bias. Nevertheless, we found few differences between people who participated in the follow-up and those who did not, as previously published [10], and thus, the possible participation bias would be minimal. While using an untargeted metabolomics approach provides a comprehensive overview, it is inherently less sensitive and specific than targeted analyses, which may impact the precision and reproducibility of the results. Finaly, the Di@bet.es Study administered a qualitative FFQ, which has been previously used in a population-based study in southern Spain [12, 45]. We are aware that ad hoc validation remains ideal. Nevertheless, the use of FFQs in the Di@bet.es study without specific ad hoc validation for the Spanish population is supported by robust scientific evidence and methodological strategies to address applicability and recall bias [46,47,48].