Nickel is a transition metal with the divalent cation Ni2+ (Ni (II)) as the most common oxidation state. Evidence exists that in biological matrixes and food it occurs primarily in the form of complex bound organic nickel, with possibly different biological properties than inorganic nickel compounds (EU RAR, 2008). Nickel may also be present in the environment in particulate form, including nanoparticles (EFSA, 2020). However, information on nickel speciation in food is limited and toxicological data are based on soluble inorganic salts (e.g. nickel chloride or sulfate) as test compounds.
Nickel can enter food chains by several pathways, including uptake by plants and accumulation in some sea molluscs. Migration from cookware, tableware, and other food contact materials results in another source of nickel in the diet. Nickel amounts to 8–10% by mass in good quality food grade stainless steel, which has a limited metal release; however, leaching of nickel into food may not be negligible for food contact materials made of poor quality stainless steel or of other metal alloys containing nickel (EFSA 2015).
In humans, the oral bioavailability of nickel depends on chemical speciation and the associated solubility of the nickel compounds as well as the fasting state of the subject. An absorption of around 10% is considered representative of dietary exposure via food and beverages for the risk characterisation of chronic oral exposure (EFSA 2020).
After absorption, nickel is widely distributed in the organism and excreted mainly via the urine; an elimination half-life of 28 ± 9 h was estimated in human volunteers. Data in rodents show that the metal is able to cross the blood brain barrier and the placenta; furthermore, it can also be excreted in breast milk (EFSA 2015).
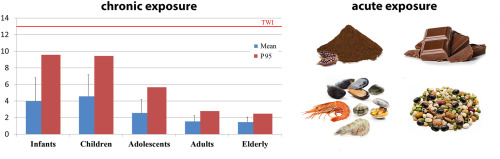
The toxicity of nickel is mediated by oxidative stress eliciting an increase of reactive oxygen species (ROS) in tissues; in addition, it has been postulated that nickel might exert some of its effects via perturbation of iron homeostasis (EFSA 2020). Due to its toxicokinetic and toxicodynamic features, nickel may affect a number of tissues and oral studies show adverse effects on liver, kidney, bone, gut microbiota, the nervous and reproductive systems. Nickel is also genotoxic, likely due to indirect effects including inhibition of DNA repair and ROS production. However, developmental toxicity is observed at lower dose levels compared to other adverse effects and for risk characterisation of chronic oral exposure to nickel the EFSA CONTAM Panel selected the increased incidence of post-implantation loss in rats as the critical effect (EFSA 2015); this choice was reconfirmed in the latest assessment, recently submitted to public consultation, where a BMDL10 of 1.3 mg Ni/kg bw per day was selected as the reference point for the establishment of a tolerable daily intake (TDI) of 13 μg/kg bw (EFSA 2020). A few recent epidemiological studies support an association between nickel exposure and adverse reproductive and developmental outcomes in humans (Chen et al., 2018; Zafar et al., 2015; Zhang et al., 2019; Ni et al., 2018).
Nickel may raise concerns also because of acute effects. Binding of nickel to proteins is responsible for the induction of allergic reactions; moreover, nickel also has a non-specific pro-inflammatory activity. Even though allergic reactions to nickel predominantly occur after skin exposure, oral intake may elicit eczematous flare-up reactions in already sensitised individuals. Accordingly, the EFSA CONTAM Panel identified systemic contact dermatitis (SCD) as the critical effect for the risk characterisation of acute oral exposure to nickel. Since a BMDL could not be derived from the available studies, the lowest-observed-adverse-effect-level (LOAEL) of 4.3 μg Ni/kg was selected as the reference point in combination with the margin of exposure (MOE) approach for characterising the risk, where a MOE of 30 or higher was considered as being indicative of a low health concern (EFSA 2020).
The purpose of Total Diet Studies (TDS) is the assessment of the exposure to contaminants and/or intake of nutrients by the general population, usually at the national level, and the characterisation of the associated public health risks (EFSA, FAO, WHO 2011; Moy and Vannoort, 2013). A TDS consists in the selection, collection and analysis of commonly consumed food purchased at retail level, processed as for consumption, and pooled into representative food groups for which consumption data are available. TDS are designed to cover the whole diet and to measure the amount of each substance ingested at the population level, providing average and high level exposure data, thus enabling to characterize associated risks by comparison with Health Based Guidance Values (HBGV) and assess the impact on public health. Therefore, TDS are an effective approach in order to obtain robust intake estimates, allowing to compare results across countries, to perform health risk assessments and to highlight issues deserving further attention by scientists and risk managers.
The 2012–2014 Italian TDS, originally launched to estimate on a nationwide basis the exposure to non-essential, toxic elements and element species (inorganic arsenic, aluminium, cadmium, lead, methylmercury, inorganic mercury, uranium) along with radionuclides (D’Amato et al., 2013; Cubadda et al., 2016), was then extended to nutrients (e.g., calcium, iron, copper, zinc, selenium, iodine) and to a number of other contaminants, i.e., other toxic elements (e.g. nickel), polychlorinated dibenzodioxins, polychlorinated dibenzofurans, dioxin-like polychlorinated biphenyls, non-dioxin-like polychlorinated biphenyls, and mycotoxins. The study was designed to provide intake estimates for the general population and selected population subgroups, both at the national level and for each of the four main geographical areas of Italy, i.e. North-West, North-East, Centre, South and Islands, since these areas may show appreciable differences, e.g. in regard of food consumption and environmental contamination patterns (D’Amato et al., 2013).
The aim of this article is to present the dietary exposure assessment of the general Italian population to nickel achieved with the 2012–2014 national TDS and to characterize the related risk in the light of the current HBGVs for chronic and acute oral exposure established by EFSA.

Dining and Cooking